Yogev Lab
The unique shape of neuronal cells is critical for their function in propagating electrical signals. Two things that make neurons so different from other cells are their size (axons can be thousands of folds longer than the cell body) and their elaborate, highly polarized morphology. Although it is clear that forming these structures and maintaining them is crucial for our brains to function, we know relatively little about the underlying cell-biological processes.



Left: pyramidal neuron drawing by Ramon y Cajal. Note how tiny the cell body is compared to the complex dendritic arbor. Middle: Electron microscopy reconstruction of synaptic vesicle precursors and dense core vesicle on microtubule tracks. Right: Artist's rendering of the middle panel. EM by Rick Fetter, drawing by Claire Richardson and Sharon Lu.
The lab studies how the neuronal microtubule cytoskeleton is formed, patterned and maintained to scaffold neuronal morphology and to support cargo delivery across very large distances. We are also interested in how transport mechanisms maintain local homeostasis at locations like synapses, remotely from the cell body.

Axonal Transport, GFP::RAB-3
Live imaging of axonal transport in an intact animal. Movie shows synaptic vesicle precursors traveling in the DA9 axon towards the synapses (left) or the cell body (right). Notice how the movement of the vesicles in punctuated by pauses. We discovered that many of the pauses occur at microtubule tips, when cargo has to switch tracks in order to continue.
Most of what we know about microtubules comes from studies of cell-division. However, unlike dividing cells, neuronal microtubules do not emanate from centrosomes. Rather, they appear as free floating polymers that tile with each to cover the length of axons and dendrites (See figure below). Even though this unique organization was described decades ago, we still don’t understand how it arises and what roles it plays in regulating the overlying transport. In the context of this overarching question, research in the lab addresses topics such as axon outgrowth and non-centrosomal microtubule nucleation, microtubule and neuronal polarity, or the regulation of transport and synapses by polymer length, abundance and dynamics.

Left: Centrosomal microtubules in a dividing C. elegans embryo (Hannak et al., 2002). Right: Neuronal microtubules. The lower panels show microtubule profiles on consecutive EM sections and a schematic of the polymers. The top panel shows the modeled EM data. Note how individual polymers can be resolved by confocal microscopy during cell division, but resolving neuronal microtubule requires EM.
A major hurdle in studying neuronal microtubules is that they closely overlap with each other, necessitating labor intensive electron microscopy reconstructions to resolve them. We developed a rapid, fluorescence based method to analyze microtubules in C. elegans neurons, thereby opening the way to genetic analysis. We can not only examine how known regulators affect neuronal microtubules, but also search for new ones. We then use double label live-imaging to follow cargo transport and how it is affected by the structure of the underlying tracks. Finally, working in vivo, in a circuit with well defined synaptic connectivity and function, allows us to probe the interplay between synaptic morphology, activity and the cytoskeleton.
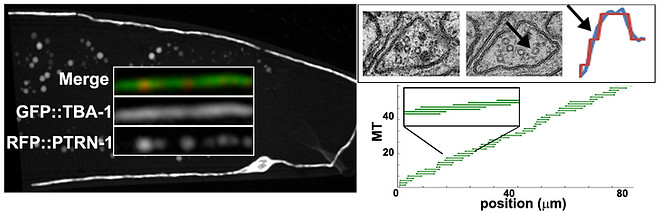
Rapid in vivo analysis of microtubule organization in the DA9 neuron. Left: Microtubules are labeled uniformly (GFP::TBA-1), and at one end (RFP::PTRN-1). A custom image analysis algorithm was developed to extract microtubule length, numbers and spacing from these images and build models like the one in the bottom right panel. Upper right panel: correlative light-EM data collected from the same axon, showing that a change in MT number seen by EM is also correctly captured by the fluorescent image-analysis tool.
We think that these questions are important because of the central role of the cytoskeleton in neuronal cell biology. Microtubules are involved in axon determination, in regulating the polarized distribution of organelles into axons or dendrites and in maintaining the composition of synapses and other outposts over the life of the neuron. Not surprisingly, mutations in cytoskeletal or transport components are often associated with neurodegeneration, and we hope that the basic insights generated from our studies will also deepen our undestanding of cellular dysfunction in disease.
